SCIENCE
ENHANCEMENT PROGRAMME |
||||||||
 |
||||||||
| quick
link: menu | intro
| P | Q
| R | S
| T | U |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Developed
by Keith S. Taber University of Cambridge
School science is not the same as ‘real’ science as experienced by professional scientists (Kind and Taber, 2005). This is obvious perhaps, but sometimes, when we are working with the school curriculum and examination specifications for so much of the time, we can easily forget this, and treat what we do in school laboratories as if it is science. Of course, professional scientists work at their science full-time, often in well-resourced workplaces, usually focussed on one small aspect of science for months (if not years), and have been well prepared for their professional role. It would be quite ridiculous to imagine a KS3 pupil expected to be able to match this level of work in science, either in terms of conceptual understanding or in conducting scientific enquiry. School science should be something that is authentic, in the sense that it reflects professional science, to give pupils a good feel for what science is about and what it involves. However, school science has to be pitched at a level that pupils can engage with successfully. So there is always a balance to be achieved in preparing a curriculum, syllabus or scheme of work. The science in the curriculum should reflect science whilst still being suitable for learners’ age, intellectual development and existing conceptual understanding. All science teachers would accept that science has to be simplified for learners. Another way of putting this is that the material specified in the curriculum is a model of aspects of science. All teachers also know that pitching material at the right level for a particular group of pupils is a skilled operation, and not always something we get right the first time. So considering the descriptions of science found in the National Curriculum as curriculum models, does not in itself tell us whether they are good models or not. The process of simplification always means that we have to leave details and complications out, and different teachers have different views about what should or could be left out in explaining key scientific ideas. My own trainee science teachers have sometimes latched onto a
term used by Jerome Bruner – intellectual honesty.
Our curriculum models need to simplify the complexity of science, but if we over-simplify, then we end up with something that no longer authentically reflects the science that we are trying to teach pupils about; it is no longer an intellectually honest model. So a good curriculum model would show the ‘optimum level of simplification’; simple enough for the pupils to understand, but still intellectually honest. To illustrate this point, consider three scientific concepts that might be modelled in the curriculum: photosynthesis, energy and ionic bonding. Photosynthesis is a very complicated process, where a thorough scientific understanding involves complex ideas about photons being absorbed and electrons being promoted in molecules. At the secondary level we do not need to go into these details – we can present a model in terms of sunlight being absorbed by a pigment called chlorophyll and this allowing a chemical change to take place. This is not a thorough model, but it is intellectually honest. Energy is a fundamental idea in science, and quite rightly considered a ‘key idea’ at Key Stage 3 (DfES, 2002). Yet, it is also a very abstract idea, so it is difficult to know how best to present it in the school curriculum. All the common approaches – the potential to do work, what we need to get things done, the ‘go’ of things – can run into problems, so there is a genuine issue of what might be the best curriculum model for the energy concept (Millar, 2003). Ionic bonding is one of the main types of chemical bond studied in secondary and college chemistry. Upper secondary pupils are often taught that the ionic bond is the transfer of an electron from (for example) a sodium atom to a chlorine atom to form ions of sodium chloride. It is common for pupils to learn that the bond is the transfer of electrons, and therefore, in sodium chloride, each ion is bonded to one other ion. For many pupils this is an idea they find difficult to overcome later if they decide to study chemistry at a higher level. Teaching secondary level pupils that the ionic bond is the transfer of an electron from a metal atom to a non-metal atom, or even just implying that the ionic bond is always formed by the transfer of an electron from a metal atom to a non-metal atom, is not only likely to mislead learners about the nature of ionic bonding, but it is actually ‘bad science’. This is a poor curriculum model because although it is simple enough for the learners to understand, it does not reflect the scientific model. To present ionic bonding in this way (which many textbooks do!) is intellectually dishonest, as it gives learners the impression they understand something, but is a false representation of the science. Any reader not convinced of this should consider how it is possible to understand the ionic bonding in a precipitate, or even in sodium chloride formed by neutralisation followed by evaporation, if the idea taught is that an ionic bond is formed where electron transfer has taken place. Here we have considered three science concepts where we present curriculum models. In one case we can reasonably simplify and produce a model of photosynthesis that is a suitable basis for understanding and further learning. In the case of energy there is still debate on how best to teach the topic and what the most suitable curriculum model is, although helpful advice is available. In the case of ionic bonding we have a curriculum model with much currency, but which is both inconsistent with science and a poor basis for progression in understanding.The road towards a curriculum model of scientific enquiry So some curriculum models do a good job for us, but others may be far from being the ‘optimum level of simplification’ that best meets pupils’ learning needs. In effect, Sc1 in the National Curriculum for Science presents us with a curriculum model of science (i.e. science as an activity) as well as various curriculum models of areas of scientific knowledge. This National Curriculum model of the nature science has two distinct components, ‘scientific investigations’ and ‘ideas and evidence’. The scientific investigations component provides pupils with a model of empirical work in science. In effect this consists of the process of planning, carrying-out and then evaluating a controlled experiment. Much empirical work in science is of the form of controlled experiment, and so to some extent the curriculum provides an authentic model. The note of caution here is that not all science works this way, as there are many sciences that use non-experimental ‘practical’ work. There is a concern then that as the controlled experiment is the form of practical work that is currently valued in formal assessments, and especially at a time when field trips seem to be under pressure in many schools, pupils in some schools may not fully appreciate that this is only a partial model of empirical work in science (Kind & Taber, 2005). The ‘scientific investigations’ thread of Sc1 is complemented by the ‘ideas and evidence’ strand. This makes up the curriculum model for understanding how scientific knowledge is debated, developed and applied. In view of the importance of teaching pupils about the nature of science, it is important that the curriculum model we present should be ‘at the optimum level of simplification’. It must make sense to pupils whilst still being an intellectually honest reflection of the ways in which science actually works. In view of the limitations of the ‘scientific investigations’ strand at modelling the way scientists undertake empirical work (think of ethologists and anthropologists for example, let alone cosmologists), it seems appropriate to consider whether the ‘ideas and evidence’ strand of Sc1 provides an optimum level of simplification of the nature of science. This was the impetus behind the Cambridge project – to explore the idea of a curriculum model of the nature of science and to help inform teachers how to go about pitching teaching about ideas and evidence in science. This was a rather ambitious aim for a modestly resourced project of limited time-span! The project set out on a path towards developing a suitable curriculum model for teaching about ideas and evidence. We would not claim to have completed that journey, but we did undertake some useful reconnaissance, and hopefully readers will find the account of our scouting parties interesting and useful when thinking about their own teaching.Models of models? Before proceeding to discuss the Cambridge project in detail, it is useful to make a point about models that could otherwise be a source of confusion. Some readers may have noticed that the curriculum model of ionic bonding criticised above was described as a model of a model (“this is a poor curriculum model because although it is simple enough for the learners to understand, it does not reflect the scientific model”). It may be suggested that models are at the very heart of the scientific enterprise and that the main ‘products’ of scientific activity are the models developed by scientists. Models are, of course, human constructions, but scientific models are constructed to represent aspects of the world. There are many different types of scientific model. These include classification systems (the five kingdoms in biology), formulae (H2O) and equations (v=u+at), schematics (typical insect body plan, or the mammalian skeleton), system diagrams (feedback cycles showing how changes in the atmosphere influence the oceans and vice versa; the water cycle), as well as scale models. These different types of models are all ways of representing something in a simplified form. In science we expect these models to reflect some aspects of the word: so the five kingdoms typology is useful if living things can usefully be considered to fit into one of the five kingdoms. To be useful the models have to both be simplifications and correspond in some way to ‘the real world’. Now some philosophers of science spend a great deal of time and energy arguing about if and how we can ever be sure there is any correspondence between our models and reality. Here we will assume that most science teachers suppose that science can be considered to be discovering knowledge about the world in which we live – that in principle scientific models and theories can reflect nature. An example: scientific and curriculum models of the ionic bond So chemists have a model of the ionic bond. (It might be more accurate to talk about sets of models at different levels of sophistication, but I’ve chosen to keep my description as simple as possible here!) For example, they might see the ionic bond as the electrical binding between oppositely charged ions in a regular lattice arrangement. This will only be a simplification of the complexity of any real structure that we would label ionic, but it is a model that is useful for many purposes. Real structures are likely to have complications, such as rogue ions, (perhaps a Mg²+ ion in an ‘NaCl’ lattice causing some distortion) and sometimes more subtle ideas from quantum chemistry may need to be considered to explain certain properties. This does not negate the model, but indicates its limitations, and its useful range of application. A good curriculum model will be an educationally appropriate simplification of the scientific model – which is itself a simplification that scientists find useful in describing and explaining certain phenomena. The notion that an ionic bond is the transfer of electrons between atoms is not a simplification of the scientific model, but a totally different idea altogether. By contrast, consider a related example. In school science we often present bonding as if it is a dichotomy (compounds have bonds that are either covalent or ionic). However, when pupils enter college they not only discover other types of bond (e.g. hydrogen bonds, van der Waals’ forces), but are also told that many bonds are best considered intermediate between ionic and covalent. This is difficult for some pupils to accept, and they find it hard to shift ideas (from a dichotomy to a continuum) that they have learnt in good faith for their GCSE examinations. At first sight this is a very similar situation to the learner who has to take on board a definition of the ionic bond as an electron transfer event, resulting in teaching at one level that needs to be ‘unlearnt’ later. Yet, teaching about the nature of science might help us here. If we bear in mind that the products of science are themselves models, and if we teach accordingly, then we may be able to help pupils deal with the apparent contradictions they meet in science classes. To present chemical bonds as either ionic or covalent (or perhaps metallic) at secondary school is a problem if the learners take this as absolute knowledge. Perhaps it would be less problematic for learners if they understood science as being largely about the development of models that are fit for a purpose, and that sometimes need to be extended or made more sophisticated. After all, many professional chemists still use the idea of ‘covalent or ionic bonds’ a lot of the time. So a curriculum model that considers bonds as ionic or covalent can be quite appropriate if it is understood as a way of classifying things that has a limit to its range of application, rather than as a ‘fact’ about nature. It can be an intellectually honest model to present in school science, but only if we teach science as a set of models rather than proven facts. So our bonding example provides us with two very different types
of curriculum model: Of course, this leads us to the very real question of whether
our pupils can cope with this image of science, or whether ‘science
as developing models of the world’ is too abstract and subtle
for them. There is plenty of research evidence that school pupils
often have quite unsophisticated appreciation of the nature of
science. This could reflect the nature of science being too subtle
or abstract for most learners of secondary age, or it may be that
such findings reflect the teaching these pupils have received.
It may simply be that the teaching they have experienced has usually
presented an image of scientists ‘uncovering truth’
and ‘proving theories’. We are left with the question
of whether seeing science as about model construction, development
and testing is too difficult to be a part of a suitable curriculum
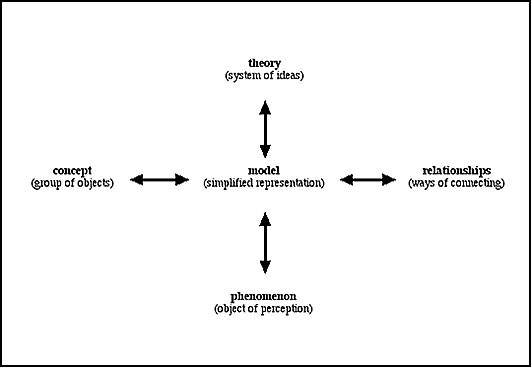
model. For example, consider Figure 1. This is a model, showing the
role of models in science. In this representation the notion of
‘model’ is related to the notion of ‘phenomena’
(that scientists study), ‘concepts’ (basic categories
we use - the ‘things’ that we perceive as making up
the world: elements, acids, insects, wasps, insulators, charges,
etc.), ‘relationships’ (such as proportion to, is
a type of, causes etc.) and ‘theories’ (seen as systems
of ideas). This is only a partial model of science, but it is
simplified representation of the central role of models in science
(Gilbert, et al. 2001). Note that one way of understanding this
figure is that models are intermediate between phenomena and theories
(as phenomena are represented in a simplified form, suitable for
incorporation into theories), and that they link concepts and
relationships (as the model shows how different conceptual entities
are linked). The double-headed arrows can also be read to imply
that phenomena, concepts, relationships and theories are all modelled
in, and can be components of models in, science.
Scientific explanations Explanations, like models, are at the very heart of science. Science is about understanding the world and that means being able to explain it. Sometimes our explanations are designed to help us predict (tomorrow’s weather), or control (destroying tumours), although understanding is sometimes seen as reward enough. We might suggest that science is about producing models that can act as the source of explanations. So, scientific explanations do not exist in isolation, but rely on scientific knowledge being applied in particular contexts. In particular, scientific explanations draw upon theories and models. A key aspiration for science education should be that learners are able to understand, and produce, explanations that we might judge to be ‘scientific’. We would like pupils to make sense of, and to actually be able to offer, ‘scientific explanations’. We would hope that pupils entering secondary school would already have some experience of working with explanations in science, and that this would develop through their schooling. Those learners who choose to study science beyond school might be expected to become adept at formulating scientific explanations.Pupils’ explanations in science However, it is always foolish to make assumptions without good grounds. So, we could ask whether we can expect learners to appreciate the nature of scientific explanations, and be able to judge whether something should be considered as ‘a good scientific explanation’, if we do not make this an explicit aim of science teaching. An analysis of explanations given by A level pupils studying
chemistry during a research project suggested that even
at this level pupils were not always able to provide good
scientific explanations (Taber & Watts, 2000). Leaving aside
questions of whether they understood the science in orthodox ways,
it was found that sometimes these pupils would use ‘explanations’
that: That advanced pupils should present such ‘explanations’
suggests that we should not assume that secondary pupils, and
especially those at KS3, will appreciate or be able to generate
acceptable explanations that meet scientific criteria. This was
a starting point for the Cambridge project. A top science set year 9 class in a city comprehensive school spent two lessons exploring the idea of explanations in science. At the start of the first lesson, the pupils were asked about their existing notions about scientific explanations. Some of these KS3 pupils were able to suggest some quite coherent and appropriate definitions:
Quite a few of the responses made reference to the explanation being based on "evidence to support it" (even "conclusive evidence") or proof, and there were quite a few references to explanations including reasons or explaining why. Despite this, quite a number of the pupils were not able to suggest an example of ‘a good scientific explanation?' Activities to develop understanding of scientific explanations The year 9 pupils were given a talk about the nature of scientific explanations, using the examples of the size of the universe and of evolution by natural selection to give a feel for the role of evidence (sometimes indirect or conditional) in scientific explanations. The view of an explanation presented was as an answer to a ‘why’ question. A good scientific explanation should be logical and draw upon accepted scientific ideas. The term ‘theory’ was used and the pupils were told that scientific theories are ideas about the world that are well supported by evidence; are internally consistent; and which usually fit with other accepted theories. The pupils were taken through a number of activities, to help them focus on the role of explanations in science. The sessions were designed to have two main pupil activities concerned with sequencing and critiquing explanations. There were also two introductory activities, which pupils seemed to quite enjoy, especially the ‘suggest an explanation’ activity. Suggesting explanationsThis was intended as a warm-up activity to introduce the notion of an explanation being a response to a 'why' question. A series of 'why' questions was prepared (appendix 1), including a number where it was not expected that the pupils would 'know' the accepted answers (thus 'suggest an explanation'). A large set of questions was prepared, so that pupils could choose questions where they either already had ideas, or where they were interested in thinking about possible reasons. Pupils worked in pairs and it was originally intended that each pair would only answer 1 or 2 questions, just to get them thinking about explanations. This was extended, as the class seemed to be enjoying the activity, producing responses that were using their science knowledge and imagination. A few examples are given here as a flavour of what these year 9 pupils produced.Why do we sweat? Explanation: We sweat because
Why don’t people lay eggs? Explanation: People do not lay eggs because
Why do only some planets have moons? Explanation: Only some planets have moons because
Why do people age? Explanation [age]: People age because
Why do some animals sometimes eat their own young? Explanation: Some animals sometimes eat their own young because
Why do we each have 2 nostrils? Explanation: We each have two nostrils because
Although many of the explanations offered were credit-worthy, they were not all adequate explanations. Some demonstrated poor logic or limited scientific knowledge. What explanations do pupils want? Pupils were also asked to report 'what are the questions you would most like to know the answers to'? This activity, along with the ‘suggest an explanation’ activity, was meant to orientate pupils to thinking about the nature of explanations. This can also be useful as a way of exploring the kind of interests that pupils have in science. The year 9 pupils were able to suggest a wide range of phenomena for which they would like explanations. The sheet, inviting pupils to offer the questions they’d most like to know the answers to can be found in appendix 2. Many would be suitable for follow-up within school science. The following list gives a flavour of their questions:
Sequencing explanations This task consists of providing pupils with the components of potential explanations (including some ‘red herrings’) and asking them to try and produce a sequenced explanation. This ‘cut and stick activity’, requires a great deal of thinking and is suitable as a small group task. The materials (appendix 3) were provided in the form of an A3 sheet with a suitable heading (a ‘why’ question) and the statements that could make up parts of an explanation. Pupils arranged the components they wished to use on the page, and added lines and additional words (‘because’) to complete the explanations. It is worth noting that this can be a very challenging task, especially when branched explanations are allowed (as in science full explanations can rely on considering several different factors). Three examples were provided, Clearly many more examples could be devised. When this work was carried out with the year 9 class, the melting example was undertaken on the OHP with the class and groups given a choice of the two other examples. Only one group completed the 'power' option. This group were able to sequence an explanation with three separate 'threads' or aspects - the greenhouse effect, the production of acid rain, and the disparate timescales for the production and use of fossil fuels. Each of the threads was relevant and logically constructed. Most groups chose to work on the question about plants, and responses of varying levels of complexity were produced. Some groups tended to produce longer, more involved explanations than others (the task certainly offers scope for differentiation). Some of the suggested explanations included connections that included quite complex sequences of statements. However, there also tended to be flaws in the logic of the explanations. It is suggested that this is an activity that pupils would benefit
from revisiting over a longer period of time, looking at different
examples (perhaps linked to different KS3 topics as they are met
in the scheme of work). Pupils were required to select examples of poor scientific explanations or good scientific explanations, and, also, to justify their choices. For poor explanations they were asked to explain the faults in each selected example, where with good scientific explanations they were asked to give an overall justification for their selection. (This approach was chosen, as it is hoped that pupils will apply a common set of criteria for evaluating explanations - the dubious examples fell down in different ways, but all the good scientific explanations would need to meet all the criteria). It was found that a number of the options selected as examples of good scientific explanations by pupils were in fact flawed. Also, some of the critiques of 'poor scientific explanations' were at the level of 'it is wrong' or 'it does not make sense'. However, there were also examples where pupils were able to offer reasons that were more specific. Again, this is an activity that is best not seen as a ‘one-off’ activity, but as an introduction to evaluating scientific explanations, to be regularly followed-up in the context of different science topics when explanations are met. Some examples of the mooted explanations that pupils were asked to consider were:
Pupils were asked to consider acceptable scientific explanations as well as suggestions that were less than perfect (with cause and effect confused; alternative options; relevant factors ignored etc). Again, this activity invites success at a range of levels, and some science content knowledge is needed to judge many of the examples. The year 9 pupils were given the full set of mooted explanations and invited to select examples of good and poor explanations, so they were not expected to be able to judge each of the ideas. Pupils’ notions of key terms in science As the work on year 9 pupils’ understanding of scientific explanations exemplifies, we need to explicitly teach about science, as well as teach science, if we want pupils to appreciate the nature of science. As part of the Cambridge project, a group of trainee teachers on the PGCE course interviewed top set science pupils to probe their understanding of some key terms in science. There is existing research in this area (e.g. Driver et al., 1996), although most of it pre-dates the introduction of the Key Stage 3 National Strategy. It was thought to be a valuable experience for trainees to find out for themselves how well KS3 pupils appreciated the use of the terms, ‘theory’, ‘hypothesis’, ‘experiment’ and ‘model’ in science. The research group interviewed a small number of pupils in three top sets and a mixed-ability group (year 7 and year 8 top set pupils at College School, Cambridge, and year 9 top set, St. John’s and year 7 mixed-ability pupils at Chesterton Community College, Cambridge. The then met at the University and shared their findings. The questions in the interviews are reproduced below, with a small selection of answers from pupils in the top set year7 group. Have you come across the word ‘theory’
in science? (and if yes)
Do you know any examples of scientific theories?
Have you come across the word ‘hypothesis’ in science? (and if yes) Can you explain what a hypothesis is?
Could you suggest an example of a hypothesis?
Have you come across the word ‘experiment’ in science? (and if yes) Can you explain what an experiment is?
Can you give any examples of experiments that scientists have done?
Can you tell me about an example of an experiment that you have done in science?
Have you come across the word ‘model’ in science? (and if yes) Can you explain what a model is?
Can you tell me about any models you have seen or used in science?
When reported in such brevity, some the responses do not give a very full idea of pupil thinking. Even so, some of these answers would make interesting starting points for a class discussion. The trainees found it very useful to talk to pupils first hand about their ideas, and this informed their own work on school placements. This is probably something that many teachers might find useful when meeting a new class, especially when unsure about how much emphasis has been placed on these terms by previous teachers. The interview schedule used by the trainee teachers can found in appendix 5.
For this a set of written probes was prepared. In each of the
probes a brief scenario was presented in terms of what people
used to think, and what scientists now think. The groups were
asked to suggest: To give a flavour of this activity, here are some examples of group responses. Scenario (Moving continents): Some people used to think that the surface of the Earth has been largely the same for thousands of millions of years. Scientists no longer think the Earth’s surface is fixed. Scientists now believe that the surface is divided into very large pieces (‘plates’) that slowly move around, so that the continents slowly change their positions.Example of a year 7 response
Scenario (The four elements): Some people used to think that everything on Earth was made of four elements called earth, water, air and fire. All materials were thought to contain a mixture of these different elements. Scientists no longer think that that earth, water, air and fire are the elements. Scientists now believe that all substances are made from a much larger number of elements (such as oxygen, carbon, hydrogen, iron, copper, nitrogen, sulfur, helium etc.) Example of a year 8 response
Scenario (Burning): Some people used to think that materials would burn (would be ‘combustible’) if they contained a special substance, phlogiston. It was believed that the phlogiston escaped during burning. Scientists no longer think that phlogiston exists. Scientists now believe that burning occurs when a substance reacts with oxygen. Example of a year 9 response
The pupils seemed to enjoy working on these exercises, and some of their suggestions are certainly creditworthy. Some of the responses produced by the small groups (e.g. “Scientists…have conducted experiments to prove it”) would, again, be a good starting point for full class discussion. There were eight topics considered in the set of probes: So can pupils appreciate the nature of science? Various research studies into learners’ ideas about the nature of science suggests many children have passed through school science lessons without acquiring a very accurate, let alone sophisticated, notion of how science occurs. The nature of science is complex, and even contentious, and any curriculum model has to be simple enough for pupils to understand, yet still be an authentic image of science. This is quite a challenge! Yet the work described above at KS3, albeit mostly with pupils in top sets, suggests that:
This small-scale exploration seems to suggest there is already
potential for pupils to make good progress in understanding the
way science operates, but as always we need to focus our teaching
on key objectives. So, for example, the meaning of a new technical
term such as ‘element’, ‘pressure’ or
‘tissue’ may be reinforced whenever it recurs, but
it may be assumed that the pupils
know what is meant by ‘theory’ or ‘experiment’,
because they seem to readily adopt the words. The trainee science teachers involved in the Cambridge project attended sessions at the University focussed on teaching about ideas and evidence, including meetings of an on-going seminar programme on ‘Meeting the Needs of the Most Able in Science’ (http://www.educ.cam.ac.uk/apecs/). They also took part in the research visits to interview top set pupils in years 7, 8 and 9. The trainees were given a range of reading material relating
to the two themes of the project (teaching about ideas and evidence
in science, and challenging able pupils). They were also presented
with a draft paper on the curriculum model of the nature of science,
describing how science ‘works’, in order to guide
their thinking about the image of science that should be taught
in school (appendix 7). The draft curriculum model, whilst a simplification,
nevertheless described a rather complex business. • To what extent they feel pupils should be taught about the nature of science as presented in the model; • How their KS3 pupils would cope with the subtlety and complexity of the image of science presented. The trainees took the ideas into their school placements and,
with the support of their school based mentors as well as faculty
tutors, tried to incorporate some work on ideas and evidence in
science into their timetabled teaching. Those who felt they were
able to produce something that colleagues may be interested in
hearing about, using or developing, were invited to submit reports
of their work. The CD-ROM includes accounts of the trainees attempts to incorporate aspects of ‘ideas and evidence in science’ in their own teaching.
DfES (2002) Framework for teaching science: years 7, 8 and 9, Key Stage 3 National Strategy, Department for Education and Skills. Driver, R., Leach, J., Millar, R. & Scott, P. (1996) Young People’s Images of Science, Buckingham: Open University Press. Gilbert, J. K., Taber, K. S. & Watts, M. (2001) Quality, Level, and Acceptability, of Explanation in Chemical Education, in Cachapuz, A. F. (ed.), A Chemical Odyssey, Proceedings of the 2nd European Conference on Chemical Education / 6th European Conference on Research in Chemical Education, Aveiro, Portugal, September 2001. Kind, V. & Taber, K. S (2005) Science: Teaching School Subjects 11-19, London, RoutledgeFalmer. Millar R. (2003) Teaching about energy, in Strengthening teaching and learning of energy in Key Stage 3 science additional support pack, Key Stage 3 National Strategy, Department for Education and Skills, pp.101-119 Taber, K. S. & Watts, M. (2000) Learners’ explanations for chemical phenomena, Chemistry Education: Research and Practice in Europe, 1 (3), pp.329-353.Appendices Please read appendices 1 to 7 which are available in Microsoft Word format, and can be opened by clicking the hyperlinks below:
|
|